Table of Contents
Brain Cancer: MRI Diagnosis and Monitoring
Brain cancer represents one of the most challenging and urgent neurological diagnoses, demanding precise imaging and multidisciplinary care. At Greater Waterbury Imaging Center, our state-of-the-art MRI protocols and experienced neuroradiology team work together to ensure accurate detection, characterization, and monitoring of brain tumors. Whether you are a patient seeking answers or a referring clinician planning treatment, this page will guide you through the role MRI plays at every stage of brain cancer management.
General Overview of Brain Cancer
Definition and Epidemiology
Brain tumors fall into two broad categories:
– Primary tumors, which arise from cells within the brain or its immediate coverings (meninges, cranial nerves, pituitary);
– Secondary (metastatic) tumors, which spread to the brain from cancers elsewhere (lung, breast, melanoma).
In the United States, approximately 24,000 new cases of primary malignant brain and spinal cord tumors are diagnosed each year. While oncologic advances have improved outcomes, glioblastoma (Grade IV astrocytoma) still carries a median survival of just 12–15 months. Brain metastases are even more common, affecting up to 30% of adult cancer patients during their disease course.
Classification and Histologic Subtypes
Gliomas
Gliomas originate from glial cells, the support cells of the central nervous system, and represent the most common primary malignant brain tumors. Within this group, astrocytomas arise from astrocytes and range from relatively indolent Grade II tumors to the highly aggressive Grade IV glioblastoma. Oligodendrogliomas, in contrast, derive from oligodendrocytes and often exhibit characteristic “fried-egg” histology; many harbor 1p/19q co-deletion, which confers both increased chemosensitivity and a more favorable prognosis. Glioblastoma (GBM), the most lethal subtype, is notorious for its rapid infiltration of surrounding brain tissue, marked neovascularity, and resistance to therapy. MRI plays a pivotal role in glioma management: high-grade lesions typically show irregular contrast enhancement, central necrosis, and significant peritumoral edema on T2/FLAIR, whereas low-grade tumors often lack enhancement and present as more circumscribed T2-hyperintense lesions.
Meningiomas
Meningiomas arise from the arachnoid cap cells of the meninges and, although frequently benign (WHO Grade I), can cause significant morbidity through mass effect on adjacent brain structures. They are the most common extra-axial primary brain tumors and demonstrate a strong female predominance—likely hormone-related—and association with NF2 mutations. On MRI, meningiomas typically appear as well-demarcated, dural-based masses with homogeneous, avid gadolinium enhancement (“dural tail” sign) and may induce hyperostosis of the adjacent skull. While surgical resection is curative in most Grade I cases, atypical (Grade II) and anaplastic (Grade III) meningiomas can recur and require adjunctive radiotherapy; advanced perfusion imaging can help predict aggressive behavior by quantifying relative cerebral blood volume.
Pituitary adenomas
Pituitary adenomas originate within the sellar region’s hormone-secreting cells and account for approximately 10–15% of primary intracranial neoplasms. They are broadly categorized as functioning (hormone-secreting) or nonfunctioning based on clinical and biochemical activity. Functioning adenomas—such as prolactinomas or growth-hormone–secreting somatotrophs—present with endocrine syndromes like galactorrhea or acromegaly, prompting early imaging. MRI of the pituitary gland utilizes thin-slice, contrast-enhanced T1 sequences in sagittal and coronal planes to detect microadenomas (<10 mm) or macroadenomas. Dynamic contrast studies—acquiring rapid, sequential images during bolus injection—can further improve detection of small lesions. Surgical resection via transsphenoidal approach remains the mainstay for macroadenomas causing mass effect, while medical therapy often suffices for prolactinomas.
Metastatic brain tumors
Metastatic brain tumors arise when systemic cancers—most commonly lung, breast, melanoma, renal cell carcinoma, and colorectal—seed the cerebral hemispheres via hematogenous spread. Metastases are diagnosed in up to 30% of adult cancer patients, and often present as multiple lesions at the gray–white matter junction, where blood flow decelerates. On MRI, metastases typically enhance brightly after contrast administration, have surrounding vasogenic edema disproportionate to their size, and may exhibit hemorrhagic or necrotic components depending on the primary histology (e.g., melanoma or renal cell carcinoma). Advanced perfusion and spectroscopy can aid in distinguishing metastases from high-grade gliomas; diffusion-weighted imaging helps identify cellularity and differentiate from abscesses. Treatment planning relies heavily on precise MRI mapping to guide stereotactic radiosurgery, whole-brain radiation, or surgical resection based on lesion number, size, and patient performance status.
Risk Factors and Etiology
– Genetic Syndromes: Neurofibromatosis types 1 & 2, Li–Fraumeni syndrome, Turcot syndrome.
– Environmental Exposures: High-dose ionizing radiation (e.g., prior cranial radiation therapy).
– Emerging Research: Investigational links to viral exposures and occupational hazards remain under study.
Clinical Presentation
Symptoms vary by tumor size and location but often include:
– Headache: New-onset or progressively worsening, frequently worse in the morning.
– Seizures: Focal or generalized.
– Focal Deficits: Weakness, speech difficulty, vision changes.
– Cognitive/Behavioral Changes: Memory loss, personality shifts.
Prompt recognition of these warning signs is critical—MRI can clarify whether they represent a neoplasm, vascular lesion, or inflammatory process.
General Description of Neurological MRI for Brain Cancer
MRI Protocols and Patient Preparation
Contrast Use: Gadolinium-based agents enhance tumor delineation. We adhere to strict screening protocols to ensure renal and allergy safety.
Positioning & Coils: High-resolution head coils and patient immobilization maximize spatial resolution.
Standard MRI Sequences
– T1-Weighted (Pre- and Post-Contrast): Defines anatomy and highlights breakdown of the blood–brain barrier.
– T2-Weighted & FLAIR: Sensitive to edema and non-enhancing tumor infiltration.
– Susceptibility (T2*): Detects hemorrhage, calcification, and treatment-related microbleeds.
Advanced MRI Techniques
– Diffusion-Weighted Imaging (DWI): Elevated cellularity in high-grade tumors produces restricted diffusion.
– Perfusion MRI: Measures relative cerebral blood volume (rCBV) to estimate tumor angiogenesis and grade.
– MR Spectroscopy: Noninvasively assesses tumor metabolites (e.g., choline/N-acetylaspartate ratio) for added diagnostic confidence.
– Diffusion Tensor Imaging (DTI): Maps white-matter tracts to guide surgical resection while preserving function.
– Functional MRI (fMRI): Localizes speech and motor areas when lesions affect eloquent cortex to inform surgical planning.
How MRI Is Used to Diagnose and Monitor Brain Cancer
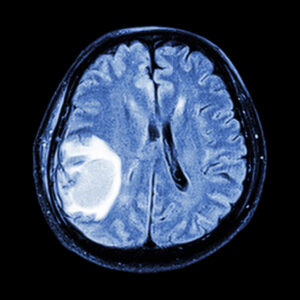
Initial Diagnosis and Characterization
MRI’s exquisite soft-tissue contrast allows differentiation between tumor types and grading features, guiding the neurosurgeon toward biopsy targets and helping pathologists choose the most informative specimens.
Pre-Treatment Staging and Surgical Planning
Three-dimensional MRI reconstructions facilitate neuronavigation, ensuring maximal safe resection. Vascular MRI sequences delineate feeding vessels, reducing intraoperative bleeding risk.
Assessment of Treatment Response
– Post-Operative MRI (within 24–72 hours): Confirms extent of resection versus expected post-surgical changes.
– Radiation Follow-Up: Perfusion and spectroscopy help distinguish true progression from “pseudoprogression,” avoiding premature changes in therapy.
– Chemotherapy Monitoring: Serial volumetric measurements track tumor shrinkage and detect early recurrence.
Long-Term Surveillance and Recurrence Detection
The standard practice is to have an MRI every 3–6 months for the first two years, when the recurrence risk is highest, then annually thereafter. Radiologists vigilantly compare studies for subtle new enhancing nodules or increases in perfusion metrics.
Emerging Applications in Research and Clinical Trials
– Radiomics & AI: Algorithmic extraction of image features can predict molecular subtypes and patient outcomes, supporting precision oncology.
– Molecular Imaging Probes: Investigational agents targeting tumor-specific receptors are under development to improve early detection and assess therapeutic response beyond anatomic changes.
For deeper insights into other neurological conditions like Alzheimer’s, dementia, stroke, multiple sclerosis, or brain infections, visit our neurological Brain MRI page.
GWIC is committed to delivering an exceptional MRI experience. We’re here to partner with you every step of the way, leveraging MRI’s full power to improve brain cancer care. Please contact us for all your MR imaging needs, including MRI for brain cancer.