FDA Recommends MRI of the Breasts for Implant Evaluation
The FDA has recommended an MRI of the breasts with implants every 3 years to check on implant integrity, to evaluate for post-implantation complications, and to evaluate the breast tissues for disease, namely breast cancer. The following article discusses the reasons for using MRI to evaluate breast implants with the primary focus on implant integrity evaluation.
MRI Breast Implant Case Study
History: Breast implant status, personal history of malignant neoplasm of breast, genetic susceptibility to malignant neoplasm of breast, acquired absence of bilateral breasts and nipples
Technique: Multiplanar images of the breast were obtained at 1.5 Tesla on a dedicated breast coil without contrast administration
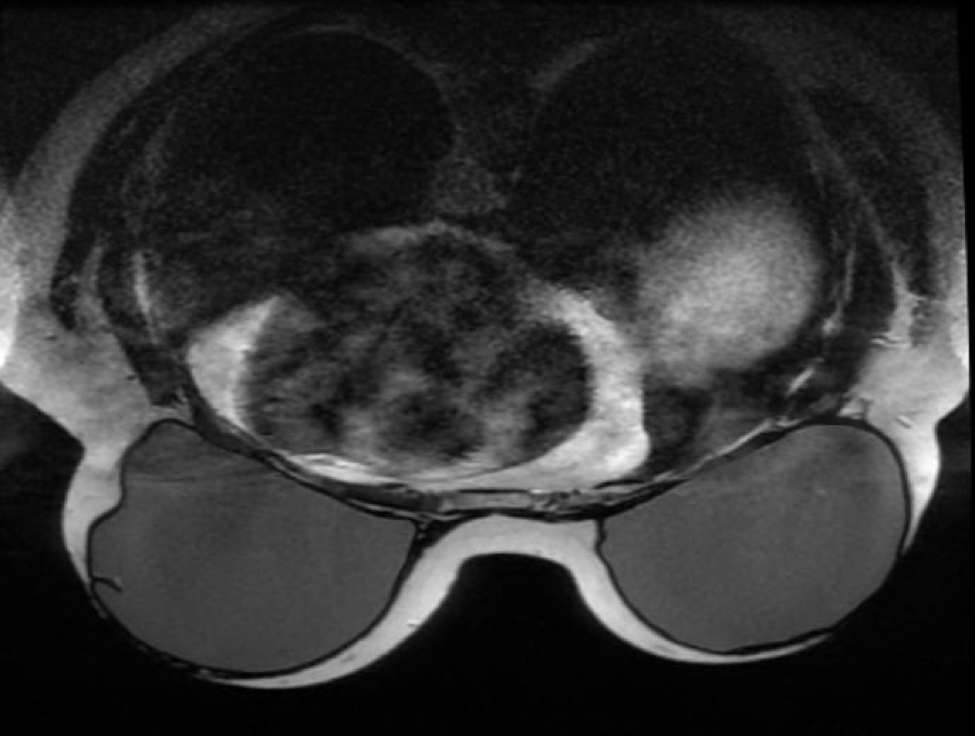
Findings: The patient is status post bilateral mastectomy, with placement of bilateral subpectoral silicone breast implants. The implants remain intact. No evidence of extracapsular spread of silicone. No evidence of a soft tissue mass. There is no axillary adenopathy.
Impression: Status post bilateral mastectomy, with reconstruction bilateral subpectoral silicone breast implants. The implants remain intact.